Neurodegenerative Research
Our research focuses on TDP-43, the major pathological protein in frontotemporal dementia and motor neuron disease. TDP-43 has also been shown to be a secondary pathology in ~50% of those with Alzheimer’s disease. Our goal is to understand the functional relationship between TDP-43 and memory, anxiety, and sociability. We are also interested in the role TDP-43 plays in plaque deposition and tau aggregation associated with Alzheimer’s disease.
Other current research interests include:
Our lab has a strong commitment to understanding mechanisms associated with neurodegeneration in Alzheimer's disease. We continue to strive and work with a deep dedication for research in hopes that one day we will live in a world without Alzheimer's Disease.
Funding: National Science Foundation (NSF)(The Acquisition of Seahorse to measure Mitochondrial Metabolism will fuel Biological Research at an HBCU). National Institutes of Health (NIH): National Institute on Aging(NIA) and National Institute of General Medical Sciences (NIGMS); Alzheimer's Association and the State of Delaware.
Other current research interests include:
- Age-dependent changes in mitophagy, mitochondrial dynamics, and metabolism associated with neurodegeneration.
- Mouse models of frontotemporal dementia and motor neuron disease.
- Developing neuroprotective targets and exploring repurposed drugs that ameliorate aggregated TDP-43 for the treatment of AD, ALS, & FTD.
- Mechanisms of cell and non-cell autonomous neurodegeneration.
- Inducible TDP-43 expression and the functional relationship between memory, network connectivity, and pathology.
Our lab has a strong commitment to understanding mechanisms associated with neurodegeneration in Alzheimer's disease. We continue to strive and work with a deep dedication for research in hopes that one day we will live in a world without Alzheimer's Disease.
Funding: National Science Foundation (NSF)(The Acquisition of Seahorse to measure Mitochondrial Metabolism will fuel Biological Research at an HBCU). National Institutes of Health (NIH): National Institute on Aging(NIA) and National Institute of General Medical Sciences (NIGMS); Alzheimer's Association and the State of Delaware.
TDP-43 & Alzheimer's Disease
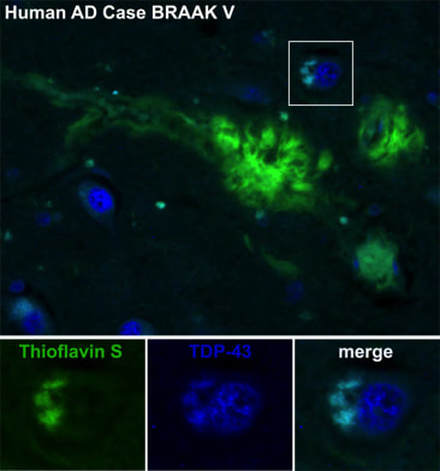
Cytoplasmic TDP-43 colocalizes with Thioflavin S (beta sheet-rich aggregation maker) in human AD cases. Immunofluorescence staining probed with total TDP-43 (N-terminal, rabbit polyclonal, 647nm, blue), Thioflavin S (488nm, green) stains aggregated proteins (plaques, tangles, etc) of Hippocampal sections from Definite AD, BRAAK stage V cases(Davis et al., 2017).
|
In pathologically severe cases of AD, we show that endogenous TDP-43 does not become entirely depleted from the nucleus as described previously. During the formation of TDP-43 cytoplasmic inclusions, nuclear TDP-43 appears to be maintained in some of the neurons near A-beta plaques (Davis et al., 2017).
TDP-43 has been shown to self-regulate expression by binding to its own mRNA in order to maintain homeostasis (Avendano-Vazquez et al., 2012; Ayala et al., 2011). We propose that the ability of TDP-43 to self-regulate diminishes as it accumulates in the cytoplasm to form inclusion bodies in AD and possibly other TDP-43 proteinopathies. Therefore, endogenous nuclear TDP-43 might be expected to increase expression in order to maintain homeostasis. We hypothesize that in late stages of AD, accumulation of TDP-43 in the cytoplasm may contribute to regulating APP subcellular localization and tau aggregation by increasing nuclear expression of TDP-43 (Davis et al., 2017). Avendano-Vazquez, S.E., Dhir, A., Bembich, S., Buratti, E., Proudfoot, N., and Baralle, F.E. (2012). Autoregulation of TDP-43 mRNA levels involves interplay between transcription, splicing, and alternative polyA site selection. Genes Dev 26, 1679-1684. Ayala, Y.M., De Conti, L., Avendano-Vazquez, S.E., Dhir, A., Romano, M., D'Ambrogio, A., Tollervey, J., Ule, J., Baralle, M., Buratti, E., et al. (2011). TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO J 30, 277-288. Davis, S.A., Gan, K.A., Dowell, J.A., Cairns, N.J., and Gitcho, M.A. (2017). TDP-43 expression influences amyloidbeta plaque deposition and tau aggregation. Neurobiol Dis 103, 154-162. |
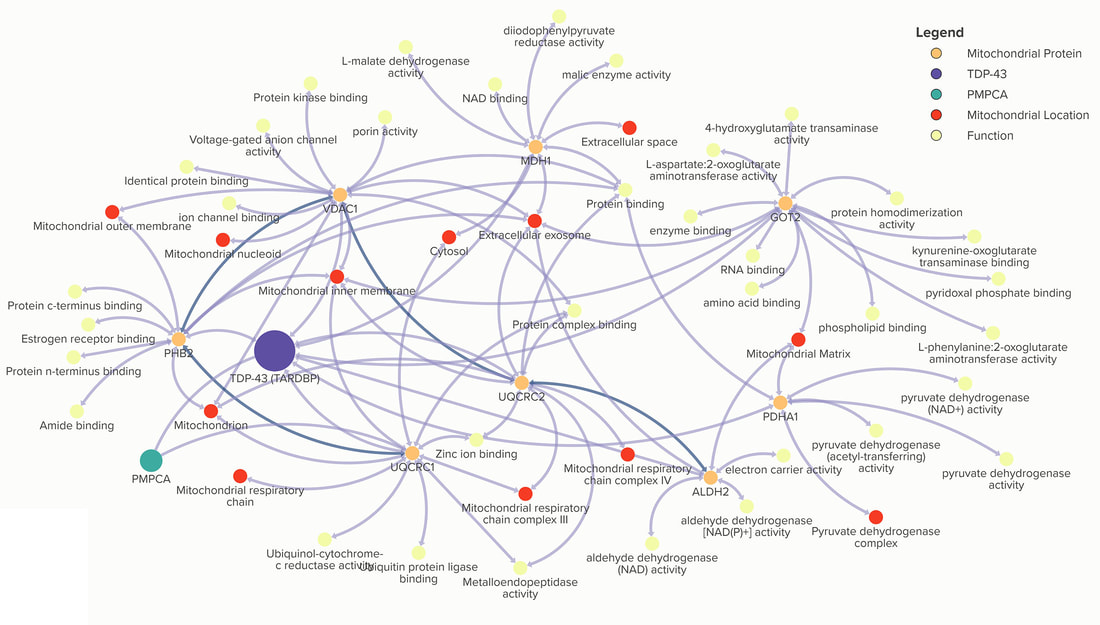
TDP-43 interacts with mitochondrial proteins critical for mitophagy and mitochondrial dynamics
Current research team.